The general consensus is that medical knowledge doubles every 18 years, while technological progress does so every two years.
Despite this rapid advancement, science has so far failed to help advance more drugs past preclinical research. On the contrary, the cost of pushing a new drug into clinical development is expected to double every nine years.
R&D teams and clinicians are increasingly concerned by the emerging rift between preclinical research and clinical testing, known as the “Valley of Death.”
This article examines how AI in the pharmaceutical industry helps bridge the gap between in vitro or animal stimulations and the clinical success of a drug candidate.
It draws a landscape of emerging AI trends in the pharmaceutical industry, biotech companies innovating preclinical research, and offers a VC market outlook to help team leaders pinpoint the highest-yield automation areas.
This post is the second article in our series on AI in drug R&D. Part 1 offers a detailed review of AI use cases in the pharma industry, top innovators, and VC market landscape in drug discovery.
Some insights featured in the article draw upon the takeaways from the AI in Pharma: The New Playbook for Drug Research & Development” webinar hosted by Ellen Knapp, Senior Intelligence Analyst in Healthcare & Life Sciences at CB Insights.
What created the Valley of Death
The deep chasm between theoretically groundbreaking biochemical research and practical improvements to clinical medicine is no news to researchers, pharma companies, or physicians.
The traditional pipeline of preclinical research, which involves gene testing in vitro followed by experiments on animal models, seems to translate poorly to human physiology. For several decades, the rate at which preclinical trials fail to apply to human clinical trials has been roughly 92%.
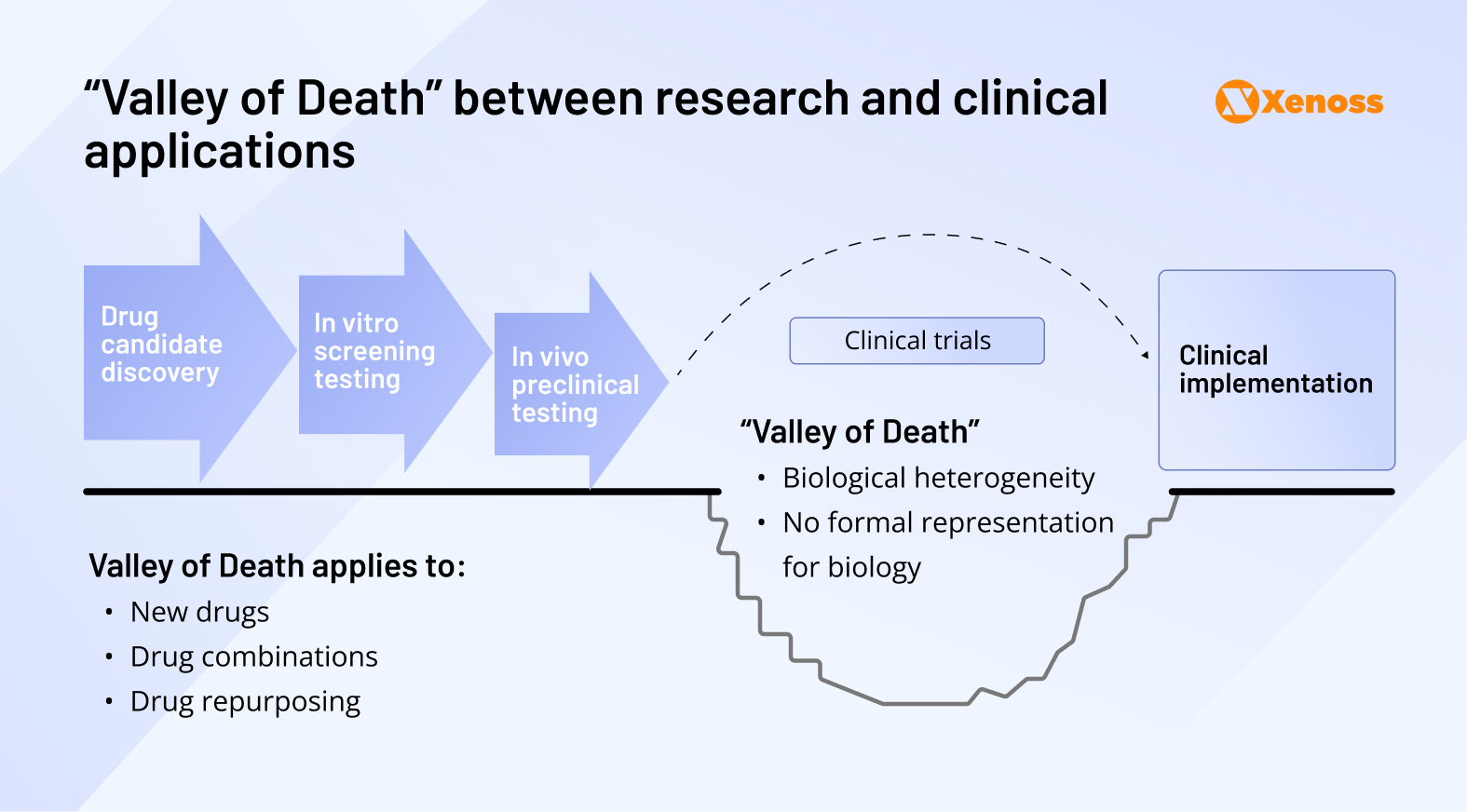
Plenty of reasons can account for such spectacular failure, from human biological heterogeneity to poor hypothesis formulation or a lack of funding and incentives, but two limitations are rooted in the pharma value chain.
Root cause #1: Limitations of animal models
The physiological differences between humans and lab animals make it nearly impossible to generate accurate drug toxicity or metabolism predictions. Drug Discovery Today points out that the failure to mirror human bodies comes at especially high costs in targeting complex therapeutic areas, like neurology or psychiatry.
Even if a close neurological match were possible, ethical concerns severely limit such research. Studies on primates, for instance, are widely discouraged. In fact, the NIH recently suspended all primate testing and retired existing test subjects to sanctuaries.
Both inherent limitations and ethical concerns around animal testing are pushing the FDA to endorse the 3R framework (Reduce, Recycle, Refine) model and try to substitute animal tests with computational analysis.
Root cause #2: Reliability and reproducibility
A paper on the challenges of preclinical research in myogenic cell therapy, published last year in Cells, attempted to reproduce the findings of 193 papers published in the research area. They found out that only four published the statistical data needed to recreate these experiments.
In 67% of cases, preclinical CROs had to modify the protocol to complete the experiments as the real-world context did not align with preclinical data, and only 41% of adjustments were feasible.
This is not an isolated case of expectation-reality misalignment.
Consider the infamous failure of Bial, touted as a first-in-class drug candidate that failed to predict neurotoxicity in animal models. That oversight sent six volunteers to intensive care, resulting in one death and four long-term neurological injuries.
More recently, the EVELYDIS clinical trial, a drug indicated for treating Duchenne muscular dystrophy and supported by seemingly impeccable pre-clinical records, recorded the death of a 16-year-old in a Phase I clinical trial.
With human lives at stake, low prediction accuracy, reliability, and reproducibility of preclinical research in drug development are alarming at least and unacceptable at most.
Can artificial intelligence in pharma bridge the Valley of Death in preclinical research?
Poor data accuracy, a lack of alternatives to animal testing, and a lack of guardrails that control the reproducibility of research before the authors share it with the rest of the scientific community seem within the grasp of machine learning.
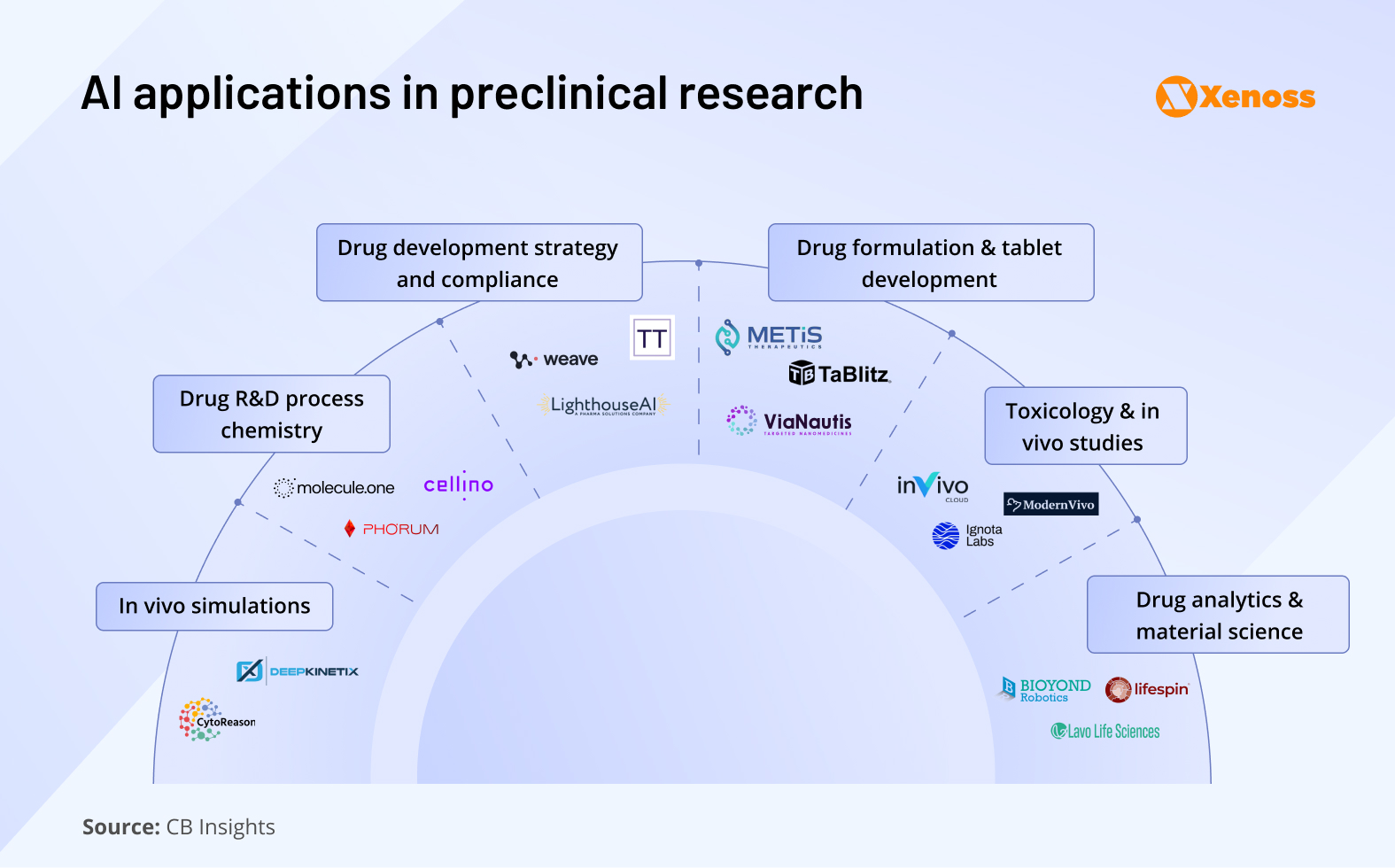
Indeed, the applications of AI in preclinical research are on the rise and cover all key areas of the pipeline:
- In vivo simulations: Testing drug effects in living organisms
- Drug R&D process chemistry: Designing and optimizing drug synthesis routes
- Drug development strategy and compliance: Automating the research pipeline and the submission of IND applications
- Drug formulation and tablet development: Creating effective and stable drug delivery forms (e.g., pills, patches, sprays)
- Toxicology and in vivo studies: Assessing safety and biological effects in animal models
- Drug analytics and material science: Analyzing drug composition and material properties
AI biotech companies are emerging in every area. Let’s zoom in on the ways in which these teams use artificial intelligence to improve the flow of preclinical testing and the milestones they have reached.
In vivo simulations

Achieving an optimal pharmacokinetic (PK) profile is essential for a drug candidate’s clinical success. Traditionally, this insight has relied on a combination of in vitro experiments and in vivo animal testing.
However, these approaches are resource-intensive and often fail to capture the synergistic effects of human physiology on drug behavior.
AI researchers are addressing the challenge by designing in-vivo simulators that predict key pharmacokinetic properties (pKa, crystal density, apparent permeability, protein unbound fraction, plasma clearance, etc.) of drug candidates based on molecular data.
Early studies show that AI-generated PK predictions closely match human-curated data, typically falling within two-to-threefold error margins, a level of accuracy that makes them viable complements to lab-based testing.
Biotech startups to watch
- Deep Kinetix creates patient-specific digital twins to simulate PK/PD responses, predict doses, and discover side effects before the drug enters clinical trials. The company’s technology ingests data from organs-on-chips (miniature systems that simulate the physiology of target organs) and enriches it with machine learning for more accurate predictions.
- CytoReason builds computational models of cells targeted by drug candidates. The company leverages AI in the medical field to analyze disease progression by combining multi-omic layers (RNA sequences, proteomics, cytometry) with natural language processing.
Drug R&D process chemistry

Optimizing drug candidates helps cut preclinical phase costs and time-to-trial compared to designing molecules de novo. While existing AI models are unreliable in creating effective molecules from scratch, they show more promise in improving the structure of shortlisted candidates.
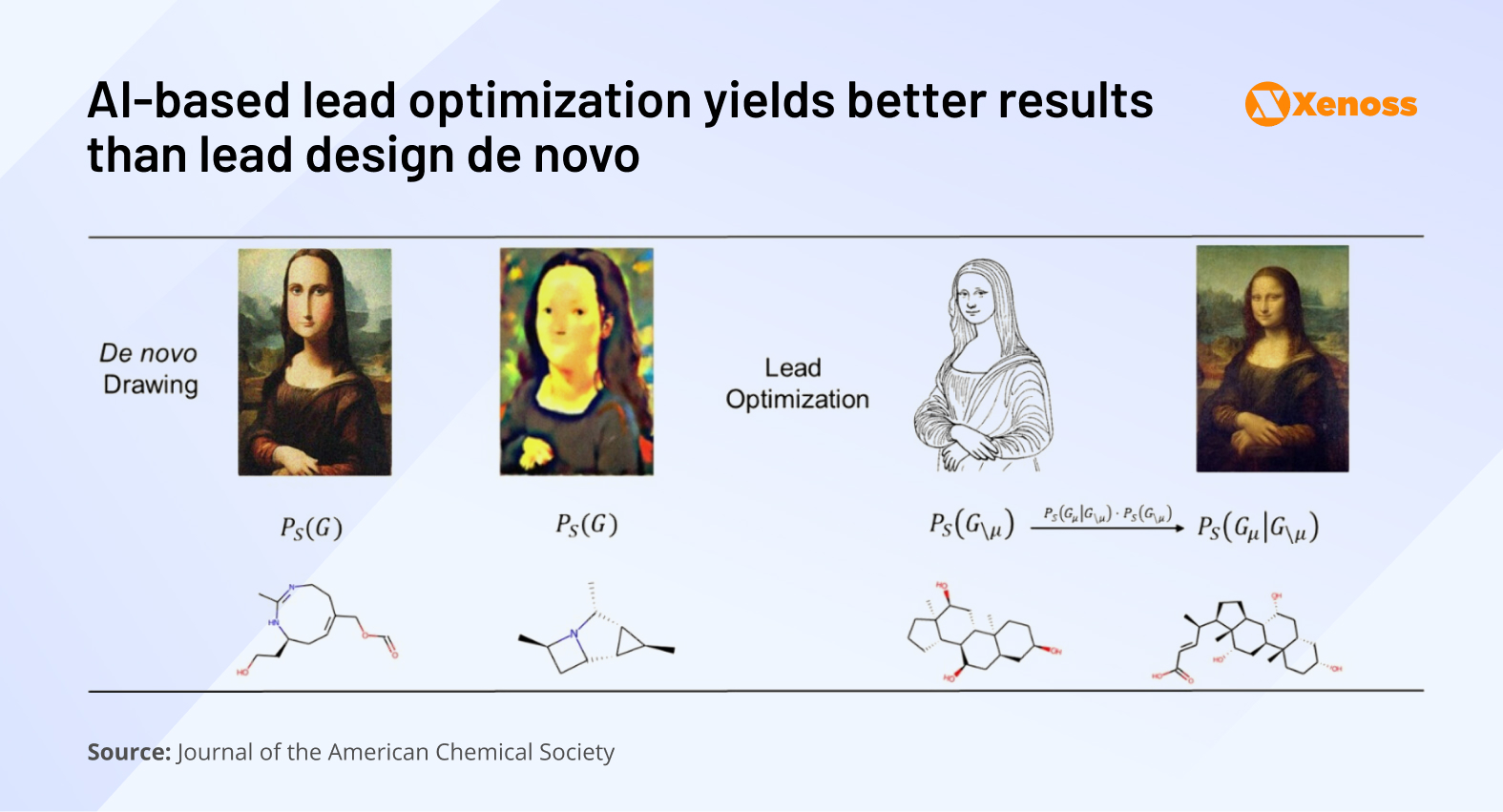
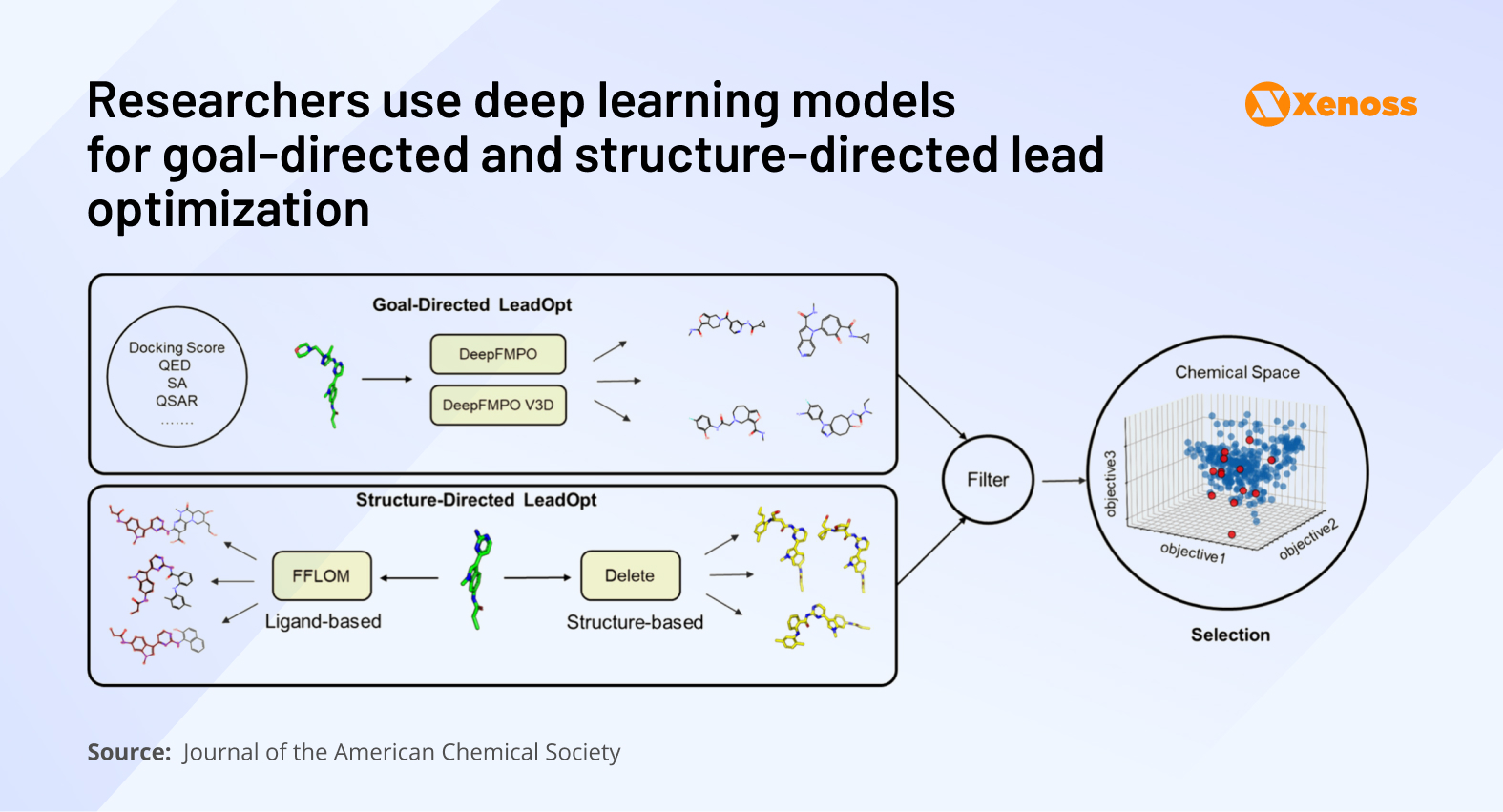
To that end, process chemists are increasingly leveraging generative AI in pharma for structure completion and property refinement. REINVENT, 15DeepFMPO, and MCMG17 are a few up-and-coming models that focus on optimizing molecular scaffolds for key performance indicators such as binding affinity, solubility, and metabolic stability.
AI medical companies to watch
- Phorum uses Phorum-GPT transformers trained in proprietary reaction and in-vitro data to predict synthesis risk, purification routes and formulation tweaks early in route-scouting.
- Molecule.one applies deep-learning retrosynthesis and condition-prediction engines trained on a real-world reaction library with over 100,000 data points to automate route selection and experiment planning for molecular chemists.
- Cellino creates digital twins to simulate biomanufacturing pipelines. It also uses computer-vision-enhanced deep learning to filter out low-quality pluripotent stem cells, driving bioprocess yield up to 80%.
Drug development strategy and compliance

Preclinical drug discovery is strictly governed by the FDA in the US and EMA (European Medicines Agency) in Europe. To keep preclinical studies compliant, researchers have to keep track of guidelines and fill out approval paperwork.
AI helps streamline these workflows by semi-automating reporting, documentation, and quality control. It can also improve the reliability and reproducibility of preclinical testing by enforcing a system of checks and balances that validates the real-world feasibility of published data.
Looking ahead, when machine learning is ubiquitous in preclinical development, AI-enabled IND platforms can share data with other solutions for AI in drug development (in-vivo stimulators, R&D optimizers, toxicology enhancers) to generate entire sections of the proposals that research teams review and submit to the FDA.
Biotech AI companies in the space
- Tehistark offers an AI medical documentation designer that reportedly cuts the time needed to draft a proposal by 50%.
- Lighthouse AI is a medical AI app that scans the FDA and DEA boards. The platform generates reports that help research teams monitor guidelines. Researchers receive real-time alerts if new regulations directly impact their projects.
- Weave helps teams auto-draft approvals, process source data with AI, and fine-tune proposal texts with generative AI.
Drug formulation and tablet development
Among all preclinical workflows, formulation and tablet development have emerged as one of the most promising and well-funded areas for AI in the medical field.
Four of the sector’s top ten VC deals in the last two years have gone to medical AI companies in this space, and more than half of the investment dollars have been awarded to the market. Machine learning is used here to optimize formulations for better bio-performance, manufactureability, and stability.
Why the surge?
Formulation design has become increasingly complex across drug classes. Biologics, for example, often require sophisticated delivery vehicles like mRNA encapsulated in lipid nanoparticles (LNPs). Meanwhile, many small-molecule drugs demand advanced formulations such as spray-dried dispersions to improve solubility and permeability.
In both cases, formulation development workflows are becoming multi-layered. Therefore, technologies that help streamline them are gaining popularity.
One standout player is Metis Therapeutics, which takes a dual-pronged approach: its AI-LNP platform targets biologics delivery challenges, while its small-molecule optimization tools address physical-chemical hurdles.
This strategy has garnered massive investor interest, making Metis Therapeutics the highest-funding biotech startup of the last two years.
Other AI medical companies reinventing formulation design
- Metis Therapeutics trains transformer models on a 10 M-compound virtual lipid library to predict ionisable-lipid and finished-LNP properties before synthesis to cut wet-lab runs and optimize AI-driven formulation design in pharmaceutical factories.
- TaBlitz supports drug manufacturing companies with an AI-powered 3-D tablet-design studio, featuring deep-learning models trained on > 160k historical designs output assessed for manufacturability, tooling life, swallowability, and dissolution for every geometry.
- ViaNautis, recently partnered with Eli Lilly to use AI in pharmaceuticals and backed by a Series A, is pioneering AI-driven polymer-nanoparticle design among other pharmaceutical industry trends.
Toxicology and in vivo studies
The traditional approach to studying adverse effects relied on initiating molecular events, such as drug-receptor interactions, analyzing the response to the event on the cellular level, and its impact on the subject’s physiological function.
This approach allowed to identify hundreds of adverse outcome pathways but the accuracy and speed of the process can still be improved.
Researchers are increasingly turning to machine learning to modernize this process, and early results are promising.
In one study, a Bayesian neural network was pitted against empirical dose-response modeling and systems biology modeling in the assessment of oxidative-stress-induced chronic kidney disease. The Bayesian neural network was more precise than the dose-response modeling and more sustainable than system biology modeling.
Similar reports of neural networks outperforming their traditional counterparts make the scientific community more accepting of using machine learning tools in toxicity prediction.
The table below reviews machine learning models currently gaining traction in toxicology.

Biotech startups to watch
- Invivo Cloud uses computer vision for “behavioral sequencing” of animal studies, simultaneously analyzing up to 100 mice. The platform supports noninvasive AI readouts of circadian rhythm, activity, and stress, feeds PK/PD dashboards.
- Ignota Labs combines cheminformatics and bioinformatics to discover new toxicity pathways and brainstorm resolution mechanisms. The platform deploys graph neural nets and transformers to trace the causes of early adverse effects before they spiral into late-stage organ failures.
- ModernVivo mines thousands of papers in seconds, extracting doses, models, and endpoints to auto-draft optimal in vivo protocols. The company’s “Design Tools” use semantic search, retrieval-augmented generation, and analytics dashboards to cut study design time and support 3Rs compliance.
Drug analytics and material science

The ability to process massive volumes of data is one of machine learning’s core advantages, making drug analytics and material science ideal beneficiaries of AI in life sciences.
Researchers have long used machine learning to unify and mine complex datasets. Now, biotech innovators are advancing the field by building analytics engines for digital twins — AI-modeled simulations that reflect patient profiles and track real-time biomarker data such as blood chemistry, hormone levels, and circadian cycles.
These digital readouts offer a more nuanced understanding of how patients may respond to a drug across different physiological states, reducing clinical risk and helping optimize trial protocols before first-in-human testing.
Smart “liquid handlers” are another promising application for AI in pharma. They automate repetitive materials screen assessments and improve materials scientists’ productivity.
AI drug development companies in the space
- BioYond Robotics combines robotics, automation, and data engineering to clean, fuse, and interpret high-throughput data for over 100 pharmaceutical teams.
- Lifespin created an AI agent that delivers differential diagnosis and drug-response analytics at scale and streams actionable, ready-for-R&D application results to dashboards and APIs.
- Lavo Life Sciences combines quantum-chemistry simulations with deep-learning ranking to screen and score lattices ≈100× faster than legacy CSP workflows.
AI innovation in preclinical drug development is still in an early stage
Pre-clinical research protocols undergo strict regulatory scrutiny, unlike drug discovery workflows that are not rigidly defined and offer R&D teams and tech innovators more room for maneuver.
To initiate human trials, the FDA mandates a full toxicology report, safety pharmacology assessments (for cardiovascular, respiratory, and nervous systems), genotoxicity testing, and comprehensive ADME/PK profiles. These requirements demand high data fidelity and explainability, raising the bar for deploying machine learning tools.
As a result, most biotech startups in preclinical AI drug development remain in early-stage funding rounds, with investors waiting for stronger proof of real-world validation.
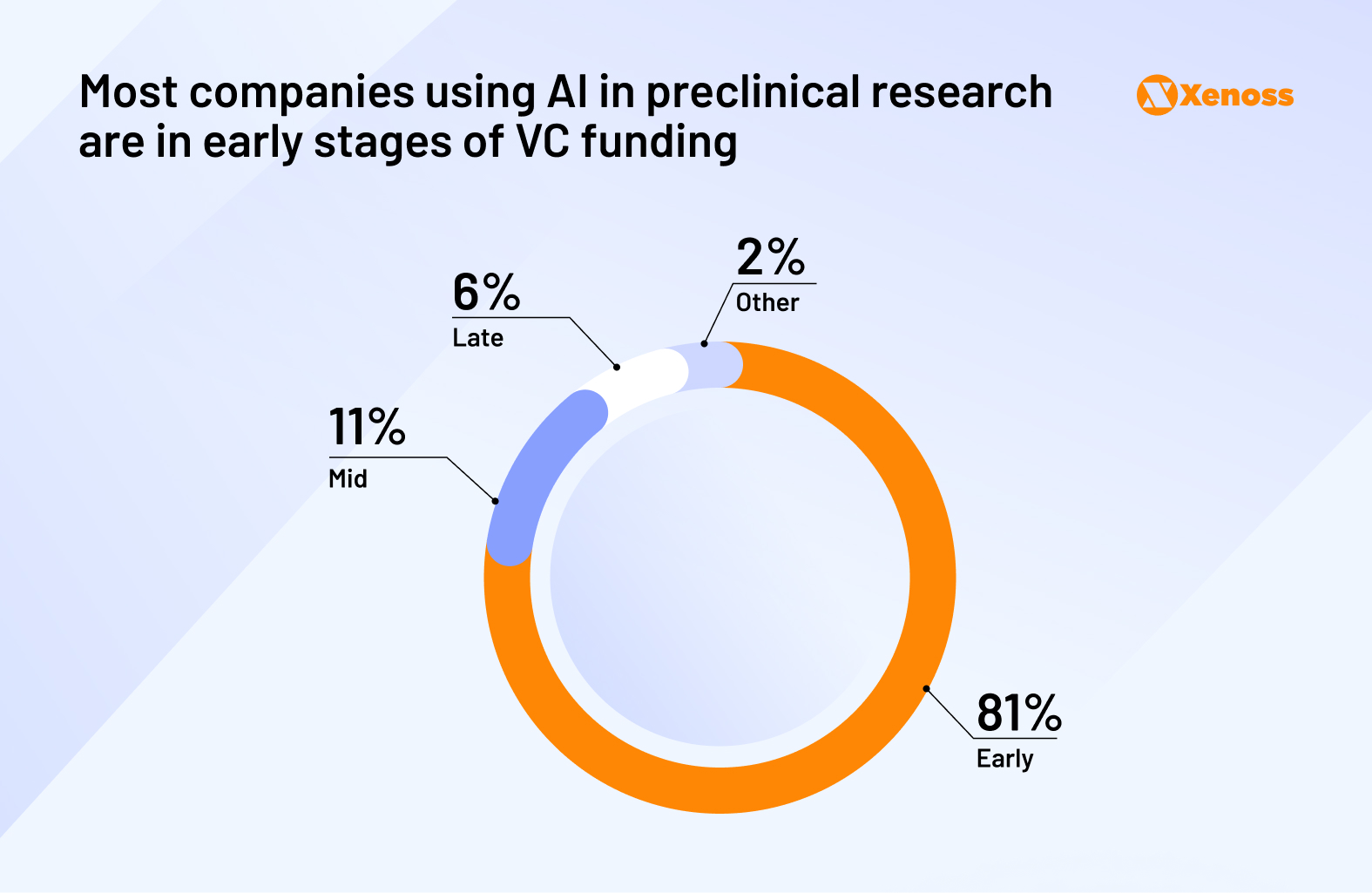
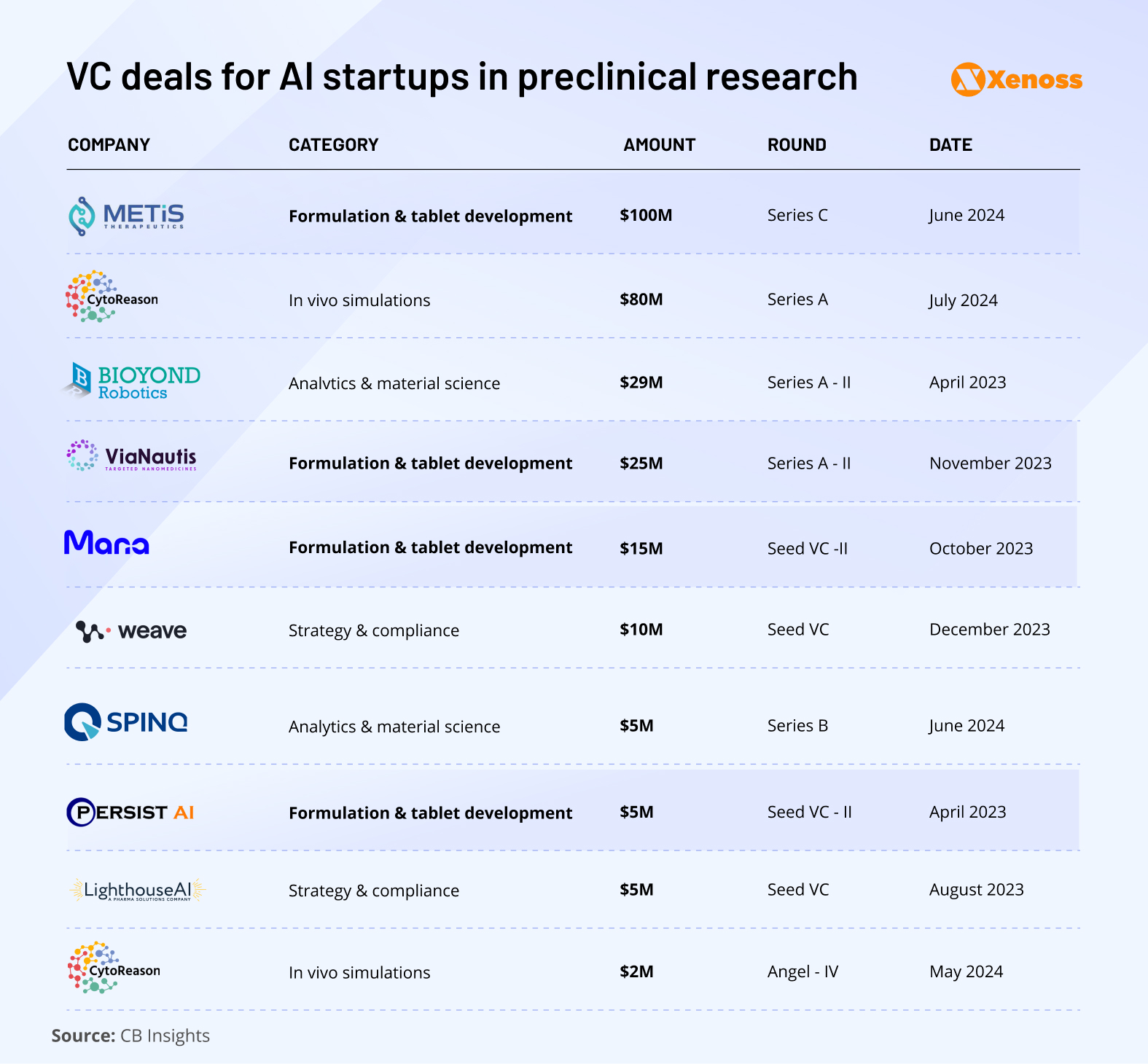
Among the exceptions, formulation development stands out as the most rapidly maturing vertical in the AI pharma market. New pharmaceutical companies like Metis Therapeutics, ViaNautis, Mana, and PersistAI have all raised Series A to C rounds over the past three years.
Other areas, including compliance automation, material science, and in vivo simulations, are also gaining momentum. Together, these three verticals have attracted over $150 million in VC funding since 2022, indicating rising investor confidence across the preclinical AI landscape.
Bottom line
Preclinical research is a critical bridge between the highly theoretical drug discovery pipeline and the high-risk testing of a drug candidate in clinical settings.
It is crucial that, by the end of this stage, drug candidates have a full outline of PK/PD properties, predictable safety profiles, optimal formulations, and defined posology.
Preclinical tests don’t offer sufficient breadth of insight, especially in the R&D workflows of drugs targeting the nervous system. Physiological differences and ethical dilemmas corner researchers into settling for imperfect models and risk eyebrow-raising failures in a clinical setting.
AI in biotech can be a reliable bridge across the bench-to-bedside gap by ensuring that, at the time of a patient’s contact with a drug, there are no grey areas in PK/PD profiles and side effects.
Generative pharma AI, deep lead optimization, and neural networks applied to toxicology all aim to increase the R&D team’s knowledge of the drug before it enters clinical trials. AI also brings operational benefits, cutting the time needed to submit applications and keeping track of regulatory updates.
AI in the medical field and preclinical research is still in its early innings. But with the right mix of technical talent, capital, and regulatory alignment, it has the potential to solve decades-old pain points and reshape the transition from lab to clinic in the years ahead.


